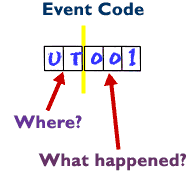
2-digit where did it happen?The "where" portion of an event is described using a process code. The process codes are generally listed in the order the process occurs in transfusion, for example:
Process Process Code Order Entry OE Sample Testing ST Unit Transfusion UT